Experience suggests it doesn’t matter so much how you got here, as what you do after you arrive.
— Louis McMaster Bujold, Barrayar
The field of assisted reproduction has advanced tremendously since Min Chueh Chang successfully performed IVF on a rabbit in 1959. Portions of pregnancy can now be safely conducted outside the human body, both at the beginning and the end. As the limit of viability is driven to lower and lower gestational ages by the tireless work of neonatal specialists and the advance of medical engineering, it seems plausible that in the near future humans might be born without pregnancy at all.
Despite being a comparatively minor achivement in medical technology, artificial wombs represent a major expansion of the available approaches to pregnancy. The opportunities afforded are valuable, and reasonably priced relative to other sorts of medical technology.
What will artificial wombs look like? And, more importantly, why don’t we have them already?
The State of the ART
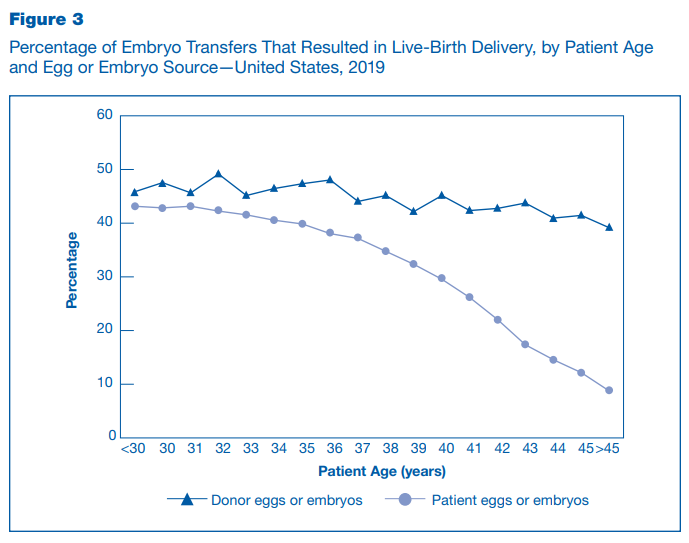
Infertility is a surprisingly common problem. 19% of women in the United States are unable to get pregnant for the first time after one year of trying, with a 26% subset having difficulty carrying to term. Assisted reproduction technology has ameliorated much of the burden associated with impaired fecundity but still requires a lot of wasted effort and low quality results that have yet to be engineered away. In 2019, the most recent year for which data is available, over 330,000 ART cycles were performed on American women. Cycles take six weeks to complete and are far from guaranteed to result in a successful pregnancy, with success rates dependent on maternal age, whether donor eggs are used, and whether the eggs are fresh or frozen.
IVF functions by directly fertilizing ova on a lab bench, allowing them to develop from zygotes into blastocysts, and depositing one or two blastocysts on the endometrium to induce implantation. The odds of this resulting in live birth range from 49.7% in women under 35 to 22.6% in women over 40. The principal reason behind the sharp decline in viability is aneuploidy – by the age of 40, around half of the remaining ova have the wrong number of chromosomes. While some forms of aneuploidy are viable, most are not, and all present the risk of complications during pregnancy. Aneuploidy explains nearly all of the variation over this time period – IVF performed using donor eggs is more consistently successful.
Embryos can be transferred to the uterus as late as the blastocyst stage, five to six days after fertilization. Waiting increases the ultimate likelihood of success, allowing for more careful discernment of embryo viability, but reduces the number of candidates to survive the culturing process.
On the reverse side, while human pregnancies average 40 weeks with ±2 weeks of variance, pregnancies as short as 28 weeks have a 90% chance of being successfully carried to term. Modern NICUs are equipped with incubators and respiratory assistance devices ranging from mechanical ventilation to ECMO, with the goal of monitoring the deficiencies particular to premature births and intervening as necessary. The limit of viability, at which point the median survival odds are a coin flip, is 24 weeks, and drops off sharply. Survival rates are only half the story, however, as premature birth is a risk factor for a dizzying variety of acute and chronic conditions.
The difficulties faced by premature infants nearly all stem from incomplete development. Incubators are necessary to correct for inability to thermoregulate, special high-calorie formula provides energy that can’t come from gluconeogenesis in immature liver tissue, and the incomplete layer of surfactant over the surface of the alveoli leads to serious problems with breathing that can be ameliorated with exogenous surfactants. Physicians in the 1940s and 1950s found out the hard way that solving this with an oxygen-rich environment interferes with the development of the retina. Premature birth is also a risk factor for disorders of all kinds later in life.
The appeal of the artificial womb is twofold: reduce the effort required for a successful assisted pregnancy, and avoid the hazards of premature birth. So, what have we accomplished thus far?
The Lamb Paper
The most comprehensive and successful attempt to date at creating an artificial womb is documented in An extra-uterine system to physiologically support the extreme premature lamb, which is exactly what it says on the tin. The brainchild of Dr. Alan Flake and a team of medical wizards at the Children’s Hospital of Philadelphia, this prototype was capable of supporting sheep embryos from 23 weeks to 28 weeks. The system consists of a polyethylene bag filled with circulating amniotic fluid, a lamb embryo with a cannulated umbilical cord, and an external oxygenation device. The lambs developed normally for 28 days – the authors claim they were viable beyond this point, but they were terminated for autopsy. Festooned with tubes and sensors, it all looks satisfyingly futuristic.
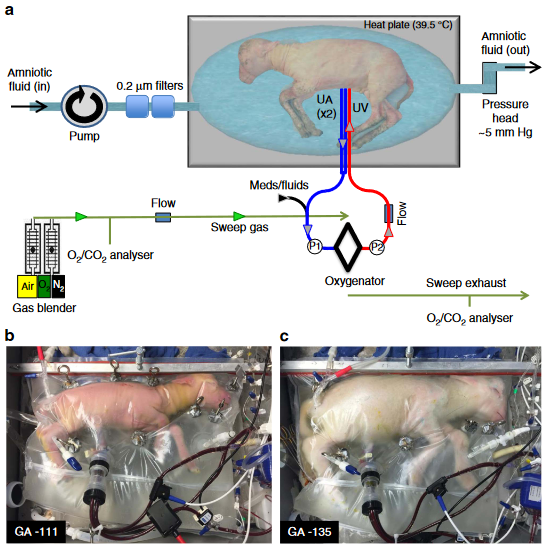
The setup is deceptively simple. Much of the lamb paper is devoted to explaining specific design choices and known pitfalls. This level of disclosure is uncommon in biology papers, and typically requires emailing the authors or their institutions to learn more. I am duly grateful. Various problems with prior attempts were successfully addressed as follows:
Embryos grown in open-circuit environments were prone to infection. The final biobag design is closed-circuit and coated with an antimicrobial film, which ultimately prevented pneumonias. Amniotic fluid was circulated at the same rate as it would in the uterine environment.
The umbilical cord undergoes vasoconstriction shortly after birth, complicating umbilical catheterization. Initial designs used the carotid artery and jugular vein, but gradually moved to using the umbilical artery and vein to avoid even worse complications. The team invented a novel catheterization technique to minimize risk of obstruction, which was ultimately successful.
Prior attempts at circulating oxygenated blood in the fetus typically caused congestive heart failure, as the fetal heart was unable to cope with the volume of circulated blood. Using a pumpless system, with the heart as the sole driver of circulation, more closely approximates the uterine environment but leads to heart failure just the same. A team from Obihiru University in 2015 attempted to solve this problem using two oxygenators in parallel and a pressure regulator, with limited success, but the CHOP team used a low-resistance pediatric oxygenator from Maquet that seems to have no issues.
While the lamb paper has some other shortcomings, in particular the inability to ascertain neurological development, the factor limiting this device’s use on fetuses of progressively lower gestational age is the reliance on the fetal heart. The youngest lambs observed in this study were unable to regulate cardiac flow well enough to avoid edema. “This suggests that there is a delicate balance between adequate and excessive circuit flow and that the ability to compensate for increased flow and supraphysiologic right atrial pressures may be dependent on developmental maturity,” the authors note, leaving the question of how to design an external pump with apposite flow control unsolved.
Nevertheless, this is a huge achievement in the field.
Failure to (Embryonically) Develop
Around one week after fertilization the human blastocyst sits within an external layer of cells called the trophoblast, which lies against the endometrium. At this point it forms a connection with the tissue of the uterus, aiming for maximum surface area to begin importing blood. The trophoblast and the highly vascular tissue it merges with later form the placenta, the source of oxygen and nutrients for the developing embryo.
This process has been studied extensively in mice. With that knowledge, Hanna 2021 and Zernicka-Goetz 2022 have made impressive strides towards growing mouse blastocysts into embryos. Yes, this machine is also satisfyingly futuristic.
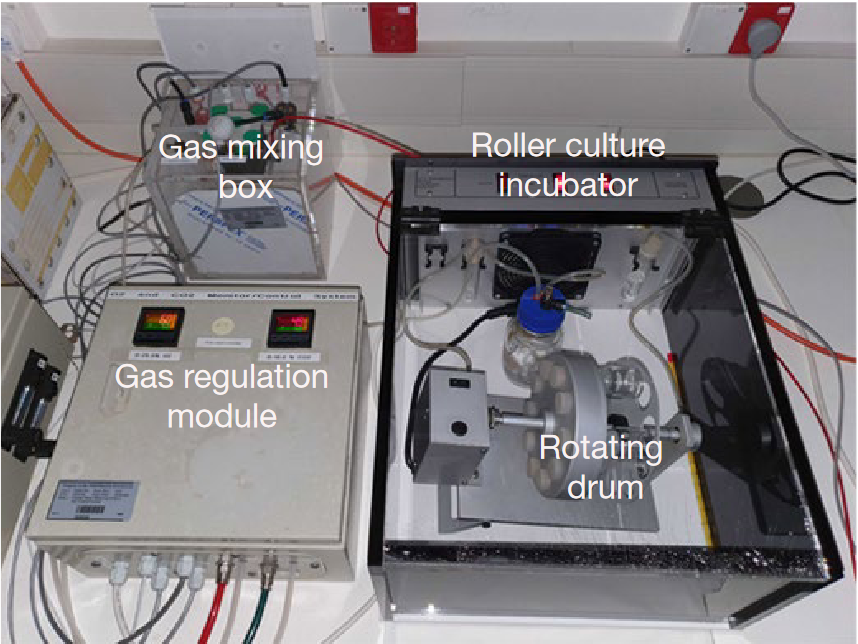
The late organogenesis stage reached with this device corresponds to the end of the first trimester in human embryology, which is too far from the fetal heart having a strong enough ejection fraction to get by with a pumpless system. The technology to bridge the first two weeks of development with the second trimester has yet to be invented, and it may be a while before we see progress.
The research thus far has been conducted on other mammals, not humans. As everyone knows, research results found in mice do not necessarily translate well to humans, although in this case we can be more specific. Human pregnancies are extraordinarily unique among animals that bear live young, involving genes and signalling pathways not seen in other animals. Human pregnancy and parturition last longer and involve more risk of injury than one would expect. As the delightfully-named Only humans have human placentas: molecular differences between mice and humans points out, mice are a reasonable approximation of humans at a gross level but only superficially similar at the genetic level.
Solving the remaining technical difficulties calls for experimenting on human embryos, which for now is where the story ends. Embryologists do not culture human embryos past 14 days, a limit from the 1980s established as law in several countries (most notably the United Kingdom and China) and as guidelines in the remainder by the International Society for Stem Cell Research.
Those guidelines have been updated as of 2021 to no longer specifically prohibit this, giving biologists in countries like America and France the freedom to experiment, but the incomplete knowledge is still a serious barrier. Details of the third and fourth weeks of development are poorly understood; the longest a human embryo has been successfully cultured is 13 days, also by Zernicka-Goetz. After that, gastrulation inevitably fails to occur. In the past year and a half, while it has technically been legal and permitted in many institutions to further study gastrulation and perhaps transcend this barrier, the scarcity of samples has prevented labs from actually doing it.
So all of this is ultimately a bit speculative. The road to the artificial womb is there, and the end is within sight, but we advance at a snail’s pace. The next step will be promulgating international standards supporting research into later stages of human embryos, helping labs to make the most of what resources and data are available. Until then, this is where we are.