Molly and Armitage ate in silence, while Case sawed shakily at his steak, reducing it to uneaten bite-sized fragments, which he pushed around in the rich sauce, finally abandoning the whole thing.
“Jesus,” Molly said, her own plate empty, “gimme that. You know what this costs?” She took his plate. “They gotta raise a whole animal for years and then they kill it. This isn’t vat stuff.” She forked a mouthful up and chewed.
—William Gibson, Neuromancer
The meat industry is one of the largest and least tractable sources of suffering on the planet. Exactly how much suffering is up to debate, but many calculations produce worryingly high estimates. Personally eschewing animal products has a marginal effect on global supply and demand, but a more measurable impact on factory farming statistics calls for a broader approach to altering the human diet. Vegan mimicry of meat products has something of a unfortunate reputation – but if the steak on your plate is made from cattle myocytes and marbled with cattle intramuscular fat, who’s going to complain?
Animal Cell Cultures Are Hard
Scientists have been able to keep animal tissue alive in vitro since 1882, when the British physician Sydney Ringer demonstrated a frog’s heart beating in a petri dish. 140 years later, cell culture is one of the fundamental tools of biology. Our ability to grow cells in a lab is used for everything from bench research to harvesting pharmaceutical products to assisted reproduction. With a little bit of tinkering, this process can be adapted to growing real meat. The first-ever synthetic hamburger was grilled in 2013 (“close to meat, but not that juicy,” according to Hanni Rützler), and a decade later Wikipedia’s Companies working on cultured meat lists dozens of startups with proof of concept.
These companies are on the verge of one of the hardest steps that any lab working with eukaryotic cell cultures will ever take: transitioning away from fetal bovine serum.
Bacterial and yeast cultures are relatively easy to keep alive. A petri dish filled with nutrient agar is virtually identical to their natural habitat. In contrast, animal cells are meant to develop in a certain context. They don’t have the ability to source nutrition from their environment, and they’re adapted to perform tasks that you would never find in a lone microorganism. Without the structure of the whole organism to support and direct them, eukaryotic cell cultures are extremely prone to giving up and dying.
Biologists in the 1910s found better luck culturing animal cells in plasma, and quickly realized that the optimal growth medium for animal cells had a small amount of embryonic serum mixed in. Better than anything discovered before or since, though, is fetal bovine serum. Myriad growth factors and a low concentration of host antibodies make it the gold standard for growing virtually every eukaryotic cell line of interest. ThermoFisher recommends using FBS for 42 out of 45 of the most common mammalian cell lines, and the remaining three still flourish the best in media made from animal serum. Unfortunately, as the name implies, fetal bovine serum is a slaughterhouse byproduct.
This is a major problem: the vat beef is still made from dead cows.
FBS is also outrageously expensive. Right now it goes for almost $2,000 US per litre at ThermoFisher and Sigma Aldritch. These are historically high prices – this not-nearly-pessimist-enough article from 2012 suggests that $250/L is an “elevated price” and that “peak serum supply may well have passed” – but the ratio of demand to supply isn’t going to decline in the foreseeable future. Research labs can and will absorb the cost, but companies that want to culture industrial quantities of animal cells have to move past using fetal bovine serum.
Chemically Defined
There are reasons to avoid FBS beyond ethics and dollars. Since it’s sourced directly from cattle embryos there’s no fixed list of ingredients, and batch-to-batch variability can make or break experiments. There are a variety of serum-free media on the market, but whether any of them will work for a particular cell line is a crapshoot. My experience is that for any given cell culture, the odds that at least one commercially available chemically defined medium will work are around 50%. Research labs use it whenever they can, because the standardization means there’s one less free variable to confound their results.
The cultured meat industry lost that coin flip. No one had found a serum-free media for culturing muscle tissue from myosatellite cells, which need a hefty dose of serum during the proliferation phase. Mosa Meat, the Dutch startup responsible for that 2013 faux-hamburger, has rolled up their sleeves and started work on a solution. Their method (open access link) involved sequencing the mRNA expressed by the myosatellite cells to determine which surface receptors were contributing to the process, followed by culturing muscle tissue in a medium supplemented with chemicals to stimulate those receptors. Mosa used DMEM/F12 supplemented with NaHCO3, a vitamin C derivative called L-ascorbic acid 2-phosphate, a growth factor called EGF1, essential amino acids, and serum albumin.
The results are encouraging! Adding transferrin and insulin to the mix helped myosatellite cells proliferate and clump together about as well as the control method. This is the best result I’ve seen thus far.
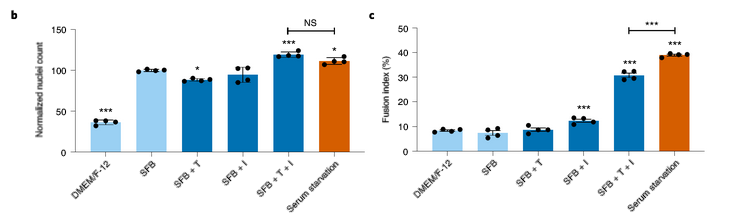
Unfortunately, the meat grown in serum-free media didn’t differentiate properly. If Mosa Meats managed to keep those cultures alive, they would’ve ended up more like beef stew than ground beef. Even the most relevant scientific protocols for culturing animal tissue using chemically defined media are unlikely to help with this, since scientists intentionally culture animal tissue in a monolayer along the faces of a vessel rather than in 3D.
The variability of FBS makes is hard to say exactly what’s missing. It has around 100 different serum proteins, transport proteins, growth factors, enzymes, hormones, lipids, vitamins, and assorted other chemicals. Mosa Meat used a handful of the likeliest candidates and did surprisingly well, but assembling all the components of FBS from scratch would be prohibitively expensive. Out of all the listed ingredients, EGF1 likely accounted for over 90% of the cost of the serum-free media Mosa experimented with, prior to adding transferrin and insulin.
The RNA-seq analysis covered the initial cell proliferation; repeating it for the differentiation phase might point towards which of these proteins would serve as the most effective media components. Unfortunately, adding new growth factors and proteins rapidly adds cost to the media. Many serum proteins are unsuitable for growing in E. coli – too many disulfide bonds, too much glycosylation, unattainable pH or temperature requirements – which calls for less efficient protein sources. Human serum albumin comes from plasma donations, but most recombinant proteins are sourced from (you guessed it) mammal cell cultures. Cost effective for therapeutic use, but less so for growing meat.
Other companies are exploring different solutions, though none of them have shared their research in any detail. Chemically defined media made using recombinant animal protein seems to be the most common approach, followed by cultivating animal tissues with less demanding growth factor requirements. Technical information from other companies in the field is sparse, and while it seems plausible that any of them might succeed (a brute force approach of starting with real FBS and working backwards by removing individual growth factors until the tissue no longer develops correctly would probably do it), there’s no guarantee any of them can do so cost-efficiently.
Ultimately Fixable
Despite the current roadblocks I’m optimistic about the future of chemically defined media for cultured meat. The expensive components of culture media have one other commonality: they’re signalling proteins, not structural ones. Growing cells need raw materials to proliferate, but the growth media simply acts as a substitute for the environment provided by the rest of the organism. The proteins bind to surface receptors on the exterior of the cells and trigger cascades of internal changes – their specific properties matter about as much as the type of metal a key is made of.
Given enough time and funding, we’ll find a way. I can imagine future synthetic meat companies engineering their own strains of muscle tissue with custom signalling pathways, relying on only one or two surface receptors that activate the required internal changes in response to cheap molecules, or even myosatellite cells that can self-assemble with no prompting necessary.
However, because the engineering cost is entirely up front, time is now the enemy. Rising interest rates and waning support from biotech investors chasing returns might impede everyone’s efforts too much to make the final product competitive with meat farmed the old-fashioned way, especially since the cost of alternatives to FBS are just a drop in the bucket compared to other economic problems holding up vat meat. Still, I think we’re giving it our best shot.

Fingers crossed.